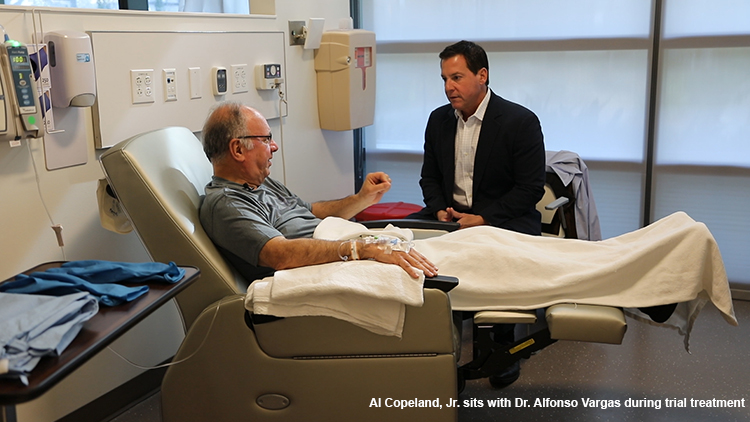
Copelands Help Save Life of Family’s Doctor
“Up to the early 2010s, this lethal variety of neuroendocrine tumors had an average survival rate of 6 to 12 months after diagnosis,” he recalls.
Vargas was diagnosed at LSU Health New Orleans, where a fellow physician from Colombia, Augusto Ochoa, MD, leads its Stanley S. Scott Cancer Center. LSU Health New Orleans recruited Dr. Ochoa from the National Cancer Institute (NCI), where he had served as Head of the Cancer Immunotherapy Laboratory of the NCI Frederick Cancer Research and Development Center.


LSU Health New Orleans Stanley S. Scott Cancer Center was selected as 1 of 11 sites to enroll patients in the second trial, and Vargas qualified. His first treatment with pembrolizumab was on November 2, 2016.
That there were so many connections and so many things had to fall into place so precisely and sequentially, it almost seems that it was meant to be.


“It’s most exciting to think that our clinicians and patients were able to contribute to approving a drug that effectively treats metastatic Merkel cell cancer, a disease for which there were few if any effective treatments,” notes Augusto Ochoa, MD, Director of LSU Health New Orleans Stanley S. Scott Cancer Center and co-Principal Investigator of the trial.
“We are excited that the Copeland-LSU Health New Orleans Partnership in Viruses, Cancer and Immunotherapy was able to be a driving force to bring the first ever Merkel cell carcinoma immunotherapy trial to the Louisiana and Mississippi areas," said Al Copeland Jr., Chairman of the Board and CEO of Al Copeland Investments.
This line of research has really taken off. The approach to managing advanced MCC has changed, with dramatic improvement in survival. Immunotherapies have provided new hope for patients with advanced Merkel cell carcinoma Both avelumab (the first immunotherapy drug approved by the FDA in 2017) and pembrolizumab are immune checkpoint inhibitors. The initial use of checkpoint inhibitors as a first-line therapy has now become the standard or care.
It’s much more effective to use immunotherapy and to use it first. MCC will recur in more than 90% of patients who are given chemotherapy, and those who have already received chemotherapy are significantly less likely to respond to subsequent immunotherapy. The researchers have found that the chance of a lasting response is about 10 times higher for immunotherapy, as long as it is given before chemotherapy. As impressive as the results have been, there is more work to be done to further improve outcomes in people with advanced Merkel cell cancer.“An effective treatment for a devastating disease such as Merkel cell carcinoma using the patient's own immune system could only be developed through many years of basic and clinical research,” adds Ochoa. “Our participation in this major discovery shows that Louisiana can be at the forefront of research and the discovery of new treatments for cancer.”
“What wonderful news it is to all the patients affected with this terrible disease, and to all the men and women who have been working for years to defeat it,” says Vargas. “We also appreciate the ACF as they have been partners in finding a possible ‘cure’ for Merkel cell carcinoma. They are now expanding their philanthropic efforts to cover more patients in the state of Louisiana and to also cover other types of cancers susceptible to treatment with this and other immunotherapies.”“The Al Copeland Foundation is proud to invest in immunotherapy and this game-changing, lifesaving treatment right here at home,” Copeland says. “While we didn’t win our father’s battle with cancer, we will win the war.”