OVERVIEW OF OUR SERVICES
The Contracts & Clinical Trials Office (CCTO) serves as a central resource for initiating contracts and conducting clinical trials for LSUHSC investigators. The CCTO encourages research and assists investigators and representatives of the pharmaceutical, biotechnology and device industries in navigating the complex research arena.
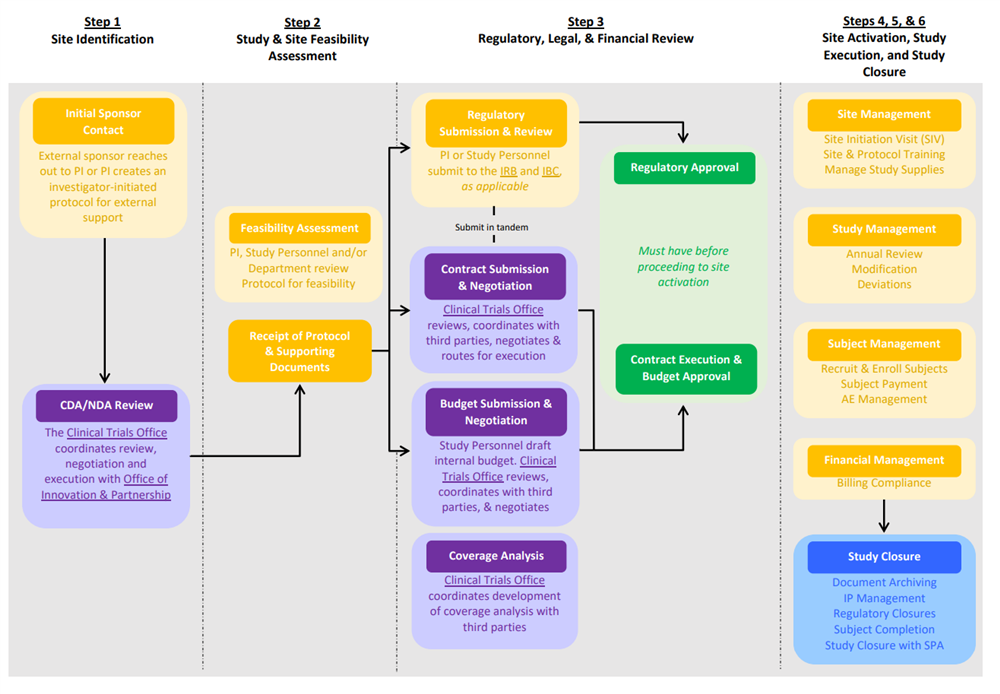
Currently, the CCTO offers select services (steps highlighted in purple) within the overall research lifecycle as shown in the workflow diagram below.
If you have any questions about our clinical research services or services provided by our affiliated institutions, please contact us at CTO@lsuhsc.edu.
Workflow for Clinical Trials at LSUHSC
|